Water, table salt, carbon dioxide, methane, ammonia, glucose, HCL, ozone, propane, and calcium carbonate are common examples of compounds.
A compound is a combination of two or more elements in which the atoms are held together by chemical bonds. In a compound, the atoms retain their properties, but they also interact with each other to produce new, unique properties. For example, water is a compound made of hydrogen and oxygen. Neither hydrogen nor oxygen exists as a liquid at room temperature, but when they are combined in the ratio of two hydrogen atoms to one oxygen atom, they form water, which is a liquid.
Examples of Compounds
There are many different types of compounds, each with its properties. Some compounds are solid at room temperature, while others are liquid or gas. Compounds can be formed from any combination of elements, including both metals and non-metals. Here are a few examples:
1. Water (H2O)
One of the most fundamental compounds, water is a life-sustaining liquid composed of two hydrogen atoms and one oxygen atom. Its unique properties make it essential for various biological processes and everyday activities.
2. Table Salt (NaCl)
Sodium chloride, or table salt, is a compound formed by the combination of sodium and chlorine. It’s not just a seasoning; it plays a crucial role in maintaining fluid balance in the body and transmitting nerve impulses.
3. Carbon Dioxide (CO2)
Produced through the combination of carbon and oxygen, carbon dioxide is a vital component of the Earth’s atmosphere. It also plays a central role in photosynthesis, the process by which plants convert sunlight into energy.
4. Methane (CH4)
Methane is a hydrocarbon compound composed of one carbon atom and four hydrogen atoms. It’s a potent greenhouse gas and serves as a primary component in natural gas.
5. Ammonia (NH3)
Comprising one nitrogen atom and three hydrogen atoms, ammonia is widely used in fertilizers and cleaning products. Its distinctive pungent odor is familiar to anyone who has encountered cleaning solutions.
6. Glucose (C6H12O6)
An essential source of energy for living organisms, glucose is a carbohydrate compound composed of carbon, hydrogen, and oxygen. It fuels cellular processes and is a key player in the metabolism of living cells.
7. Hydrochloric Acid (HCl)
A strong and corrosive acid, hydrochloric acid is formed by the combination of hydrogen and chlorine. It’s used in various industrial processes, including the production of pharmaceuticals and food.
8. Ozone (O3)
Ozone is a molecule made up of three oxygen atoms. While it exists in the Earth’s stratosphere, forming the ozone layer that protects us from harmful ultraviolet radiation, ground-level ozone can be detrimental to human health.
9. Ethanol (C2H5OH)
Commonly known as alcohol, ethanol is a compound used in beverages, fuel, and as a solvent in various industries. Its structure consists of two carbon atoms, six hydrogen atoms, and one oxygen atom.
10. Sulfuric Acid (H2SO4)
Sulfuric acid is a powerful and widely used acid formed by the combination of sulfur, oxygen, and hydrogen. Its applications range from industrial processes to the production of fertilizers.
11. DNA (Deoxyribonucleic Acid)
The building blocks of life, DNA molecules are complex compounds that carry genetic information. The combination of nucleotides containing adenine, thymine, cytosine, and guanine forms the basis of the genetic code.
12. Caffeine (C8H10N4O2)
A stimulant found in coffee, tea, and various energy drinks, caffeine is a compound composed of carbon, hydrogen, nitrogen, and oxygen. It affects the central nervous system, providing a temporary boost in alertness.
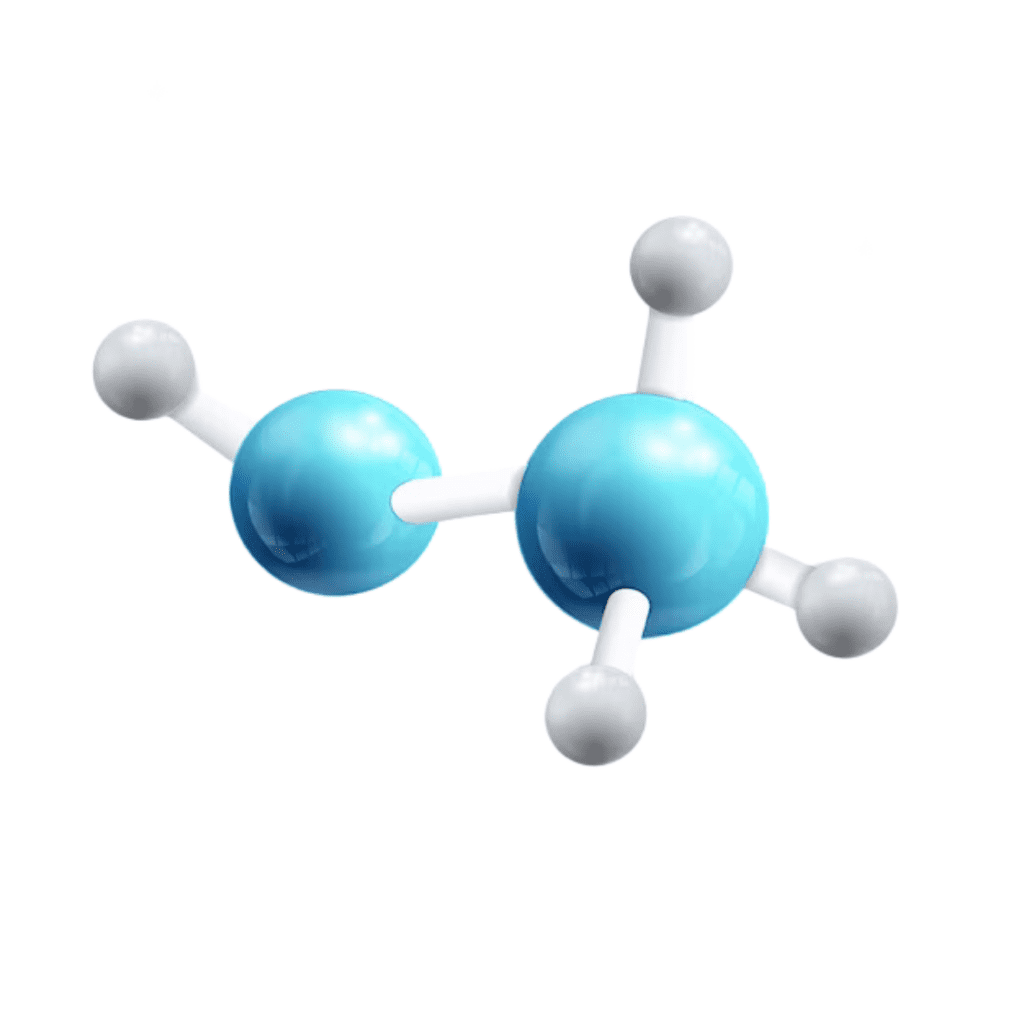
13. Propane (C3H8)
Used as a fuel for heating and cooking, propane is a hydrocarbon compound with three carbon atoms and eight hydrogen atoms. Its clean-burning properties make it a popular energy source.
14. Calcium Carbonate (CaCO3)
A compound abundant in nature, calcium carbonate is found in limestone, marble, and chalk. It’s a key ingredient in the formation of bones and shells and is widely used in industries such as construction and pharmaceuticals.
15. Chlorophyll
Chlorophyll is a complex compound responsible for the green pigment in plants. It plays a crucial role in photosynthesis, capturing light energy and converting it into chemical energy.
Chemical Bonds in Compounds
A compound is a material made up of two or more elements that are chemically bonded together. The elements in a compound are held together by chemical bonds, which are forces that hold atoms together.
There are three types of chemical bonds: ionic, covalent, and metallic. Ionic bonds form between metals and non-metals, covalent bonds form between non-metals, and metallic bonds form between metals.
Ionic Bonds
Ionic bonds occur when one atom donates an electron to another atom. This creates an electrically charged particle called an ion. Ions are attracted to each other by electrostatic forces, which hold the ions together in a compound.
Covalent Bonds
Covalent bonds occur when two atoms share electrons. The electrons are shared equally between the atoms, and the atoms are held together by electromagnetic forces. Covalent bonds can be either single or double. In a double bond, two pairs of electrons are shared between the atoms.
Metallic Bonds
Metallic bonds occur between metal atoms. The metal atoms share electrons freely, and the resulting electromagnetic forces hold the atoms together in a compound.
Synthetic and Natural Compounds
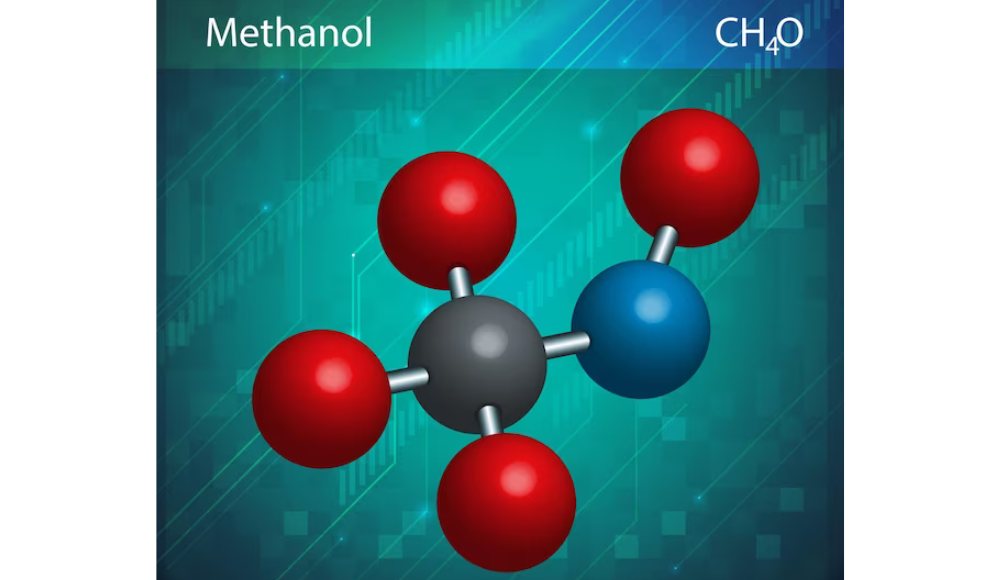
Compounds are molecules composed of two or more atoms held together by chemical bonds. There are many different types of compounds, but they can broadly be classified into two categories: synthetic and natural.
Synthetic compounds
Synthetic compounds are those that are artificially created in laboratories. They can be designed to have specific properties and functions, and as such, they have a wide range of applications. Common examples of synthetic compounds include plastics, pharmaceuticals, and dyes.
Natural compounds
Natural compounds are those that occur naturally in the environment. While they may be used for some commercial purposes, their primary purpose is not man-made. Examples of natural compounds include minerals, fuels, and food ingredients.
Examples of Commonly Used Compounds
There are many different types of compounds that are used in a variety of different contexts. Here are some examples of commonly used compounds:
- Water H₂O
- Carbon dioxide CO₂
- Methane CH₄
- Oxygen O₂
- Nitrogen N₂
There are many different types of compounds that are used in a variety of industries and applications. Here are some examples of commonly used compounds:
Acetic acid
Acetic acid is a compound that is found in vinegar. It is also used as a solvent and as an intermediate in the production of other chemicals.
Aluminum oxide
Aluminum oxide is a compound that is used in the production of aluminum metal. It is also used as an abrasive and as a catalyst.
Ammonia
Ammonia is a compound that is used in the manufacture of fertilizers and explosives. It is also used as a household cleaner.
Antimony
Antimony is a compound that is used in the production of batteries, glass, and ceramics. It can also be used as a poison.
Arsenic
Arsenic is a compound that is used in the production of semiconductors and pesticides. It can also be poisonous if ingested.
Popular Medical Compounds

Many popular medical compounds are used to treat various conditions. Some of these compounds include:
Amino acids
Amino acids are the building blocks of proteins and are essential for many bodily functions. They can be used to treat conditions such as malnutrition and muscle weakness.
Antibiotics
Antibiotics are used to kill bacteria or prevent their growth. They are commonly used to treat infections and diseases caused by bacteria.
Aspirin
Aspirin is a pain reliever and anti-inflammatory medication. It is commonly used to relieve pain, reduce fever, and decrease inflammation.
Corticosteroids
Corticosteroids are a type of steroid hormone that is produced by the adrenal gland. They are often used to treat inflammatory conditions such as arthritis and asthma.
Popular Agricultural Compounds
There are a variety of compounds that fall under the agricultural category. These include, but are not limited to: pesticides, herbicides, fungicides, and insecticides. Pesticides are chemicals used to kill or control pests. Herbicides are chemicals used to kill or control weeds. Fungicides are chemicals used to kill or control fungi. Insecticides are chemicals used to kill or control insects.
Compounds are found in everyday items like food and medicine. Knowing their characteristics and interactions is important for safe and effective production. Understanding their existence and function is essential for our safety and well-being.