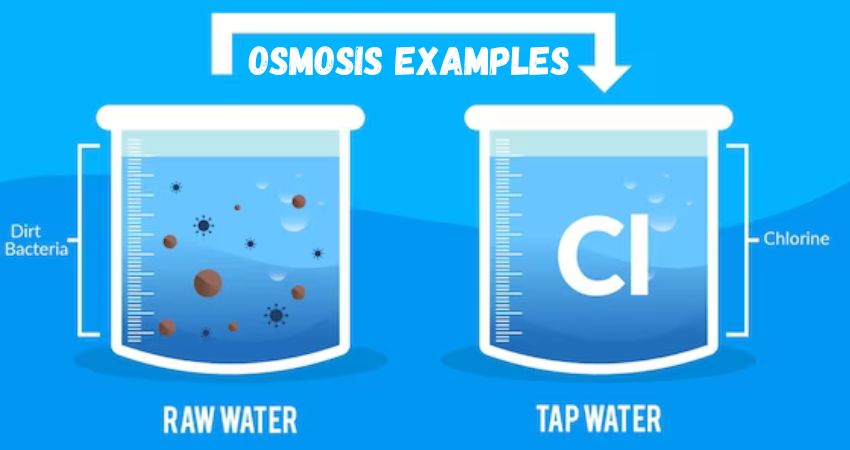
Our blog Osmosis Examples introduces you to the beautiful world of osmosis. You can discover the role of osmosis in membrane science, biology, health, energy harvesting, and cleaning, thus joining us on this journey of learning and understanding the hidden magic of osmosis.I
Definition of Osmosis
Osmosis is a fundamental biological process where molecules move from an area of high concentration to an area of low concentration through a semi-permeable membrane. This natural phenomenon plays a crucial role in various aspects of life, influencing the survival and functioning of both plants and animals.
Importance of Osmosis in Life
The significance of osmosis extends far beyond its role in basic biological processes. Understanding osmosis is key to comprehending how living organisms maintain balance, regulate cell functions, and interact with their environment.
Osmosis in Plants
The phenomenon of osmosis in plants are explained in given below;
Role in Plant’s Survival
Plants, being sessile organisms, rely heavily on osmosis for survival. The movement of water through their cells helps maintain turgor pressure, giving plants the structural support necessary for upright growth.
Process of Water Absorption in Roots
Osmosis is at the heart of water absorption in plant roots. As soil water concentration varies, roots facilitate the movement of water molecules, ensuring that the plant receives the necessary hydration for photosynthesis and other vital functions.
Osmosis in Animals
The phenomenon of osmosis in animals is given below;
Regulation of Cell’s Life
In animal cells, osmosis regulates the internal environment. Cells selectively absorb water to maintain an optimal balance, preventing them from either shrinking or bursting due to osmotic pressure.
Also Read: Examples of Green Solvents
Nutrient and Mineral Transfer in Cells
Osmosis facilitates the transfer of nutrients and minerals across cell membranes. This is essential for cellular functions, ensuring that cells receive the necessary components for growth, repair, and energy production.
Intestinal Absorption of Nutrients and Minerals
The digestive system employs osmosis to absorb nutrients and minerals in the intestines. The selective permeability of cell membranes allows for the controlled passage of essential substances into the bloodstream.
Examples of Osmosis
Examples of Osmosis are given below;
- Hydration of Dry Sponges: When a dry sponge is placed in water, osmosis causes water molecules to move into the sponge, making it swell and regain its original shape.
- Rehydration of Dried Fruits: Dried fruits, such as raisins or apricots, absorb water and swell due to osmosis when soaked in a liquid.
- Dehydration of Prunes: Conversely, prunes shrink when placed in a hypertonic solution, as water moves out of the fruit cells.
- Cellular Uptake of Water in Plants: Osmosis is responsible for the movement of water into plant cells, maintaining turgor pressure and supporting plant structure.
- Plasmolysis in Plant Cells: When a plant cell is placed in a hypertonic solution, water leaves the cell, causing it to shrink and leading to plasmolysis.
- Root Hair Cells Absorbing Nutrients from Soil: Osmosis is instrumental in the absorption of water and nutrients by root hair cells in plants.
- Grape Swelling in Wine Making: During the fermentation process in winemaking, osmosis causes grapes to swell as water moves into the fruit.
- Dehydration of Salted Meat: Salting meat induces osmosis, drawing water out of the cells and preserving the meat by inhibiting bacterial growth.
- Swelling of Raisins in Juice: Raisins left in a juice solution absorb water through osmosis, becoming plump and juicy.
- Crisping of Vegetables in Pickling: Vegetables in pickling solutions undergo osmosis, absorbing water and becoming crisp.
- Shriveling of Plant Leaves in Salty Soil: Excessive salt in soil can lead to osmotic water loss from plant cells, causing leaves to shrivel.
- Egg Osmosis Experiment: Placing an egg in a hypertonic or hypotonic solution showcases osmosis as water moves in or out, affecting the egg’s size.
- Water Absorption in Cooking Rice: Rice grains absorb water during cooking, a process driven by osmosis.
- Rehydration of Dried Mushrooms: Dried mushrooms regain their original texture and size when rehydrated in water, a result of osmosis.
- Dehydration of Saltwater Fish: Fish preserved in salt undergo osmosis, losing water and preventing bacterial decay.
- Crisping of Potato Chips: Osmosis is involved in the process of making potato chips, where water moves out of potato slices during frying.
- Plumping of Currants in Baking: Currants in baked goods absorb moisture through osmosis, contributing to their plump texture.
- Rehydration of Gelatin in Cooking: When gelatin is mixed with water, osmosis occurs, causing the gelatin to absorb water and set.
- Drying Effect of Salt on Food Preservation: Salting food items like fish or meat draws out water through osmosis, preventing bacterial growth and spoilage.
- Shrinking of Grapes in Raisin Production: Grapes lose water and shrink during the drying process, facilitated by osmosis.
- Cellular Water Balance in Animal Cells: Osmosis helps maintain the water balance within animal cells, preventing dehydration or swelling.
- Freezing Point Depression in Ice Cream Making: Adding sugar to the ice cream mix lowers the freezing point through osmosis, resulting in a smoother texture.
- Wrinkling of Skin in Saltwater: Prolonged exposure to salty seawater can cause osmotic water loss from skin cells, leading to temporary wrinkling.
- Swelling of Gel Beads in Water: Gel beads, used in gardening or as sensory toys, expand when immersed in water due to osmosis.
- Dehydration of Saltwater Algae: Osmosis plays a role in preserving seaweed by drawing out water and preventing decay.
- Rehydration of Dried Paint in Watercolor: Dried watercolor paint can be rehydrated with water, and osmosis aids in this process.
- Shrinking of Cut Flowers in Vase: Flower stems absorb water through osmosis, keeping the flowers hydrated and fresh.
- Osmosis in Human Red Blood Cells: Red blood cells maintain their shape through osmosis, ensuring proper functioning within the bloodstream.
- Dehydration of Pickles in Brine: Cucumbers in pickling brine lose water through osmosis, contributing to the preservation of pickles.
- Water Absorption in Bread Dough: Osmosis is involved in the hydration of yeast and flour particles in bread dough, influencing the rising process.
Osmosis Examples of Everyday Life
Everyday examples of osmosis are given below;
Soaking of Resins or Dry Fruits in Water
The process of soaking resins or dry fruits in water involves osmosis. Water molecules penetrate the dried fruits, rehydrating them as osmotic pressure equalizes.
Pruning or Wrinkling of Fingers in Water
The familiar wrinkling of fingers after prolonged exposure to water is a result of osmosis. The skin absorbs water, causing cells to swell and leading to the characteristic wrinkling.
Effect of Salt on Slugs or Snails
Sprinkling salt on slugs or snails induces osmosis. The salt draws water out of their cells, dehydrating and ultimately harming these pests.
Thirst Triggered by Salty Food
Consuming salty food triggers thirst due to osmosis. The high salt concentration in the bloodstream prompts water absorption to maintain balance, resulting in the sensation of thirst.
Osmosis in Membrane Science
The phenomenon of osmosis in membrane science is given below;
Separation, Desalination, Reverse Osmosis for Water Purification
Membrane science leverages osmosis for water purification. Techniques like reverse osmosis use semi-permeable membranes to separate impurities, providing clean drinking water.
Emergence of New Nanomaterials
Advancements in nanomaterials are influenced by osmosis principles. Researchers are exploring nanostructures inspired by osmotic processes for applications in medicine, filtration, and materials science.
Osmosis in Biology and Health
The process of osmosis in biology and health is explained in given below;
Kidney Filtration Process
The kidney’s filtration process relies on osmosis to selectively reabsorb water and essential nutrients while removing waste products. This ensures the body maintains a proper internal balance.
Osmosis and Energy Harvesting
The process of osmosis and energy harvesting are given below;
Osmotic Power and Blue Energy
Osmotic power, also known as blue energy, harnesses the energy generated by the osmotic pressure difference between saltwater and freshwater. This emerging field holds promise for sustainable energy production.
Capacitive Mixing
Capacitive mixing is a novel concept in energy harvesting that utilizes osmosis. It involves the controlled mixing of freshwater and saltwater to generate electrical energy through capacitive processes.
Osmosis in Detergency and Cleaning
Oil Recovery in Porous Media
Osmosis finds applications in detergency and cleaning, particularly in oil recovery from porous media. By manipulating osmotic forces, it becomes possible to extract oil efficiently from challenging environments.
This blog post highlights the diverse and fascinating examples of osmosis, illustrating its pivotal role in the natural world and its applications across various scientific disciplines. Understanding osmosis opens doors to innovations that contribute to our well-being and the sustainability of our planet.