There are numerous examples of sublimation like dry ice, naphthalene, camphor, iodine, snow and ice, frost formation, air fresheners, etc that showcase the fascinating behavior of certain materials. In this article, I am telling you about the sublimation and its examples.
Definition of Sublimation
Sublimation is a physical process in which a substance transitions directly from its solid phase to its gas phase without passing through the intermediate liquid phase. This phenomenon occurs when the ambient pressure is low enough to permit the solid substance to vaporize without melting.
- During sublimation, the solid molecules gain sufficient kinetic energy to break free from their fixed positions in the crystal lattice and escape into the gas phase.
- Sublimation is influenced by factors such as temperature, pressure, and the nature of the substance itself. This process is commonly observed in various substances, including dry ice (solid carbon dioxide), mothballs (naphthalene), and camphor, among others. Sublimation has diverse applications across different fields, including chemistry, physics, materials science, and industry, ranging from purification techniques to the creation of specialized materials and products.
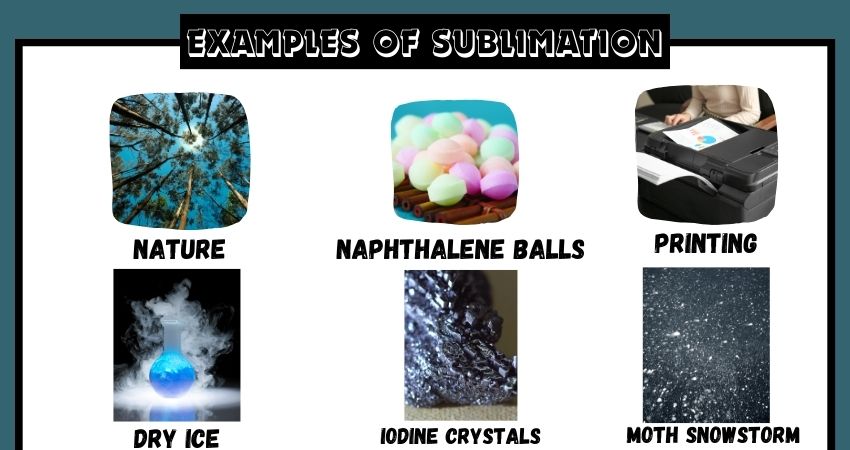
Examples of Sublimation
Sublimation finds applications in various fields, including chemistry, physics, and even everyday life. By understanding sublimation and its occurrence, we can appreciate the extraordinary properties of materials and their behavior under specific conditions. This process showcases the extraordinary behavior of certain materials under specific conditions. In this article, we will delve into the diverse world of sublimation, exploring 25+ examples that highlight its occurrence in various substances and everyday scenarios.
1. Dry Ice (Solid Carbon Dioxide)
When exposed to room temperature, dry ice sublimes directly from a solid to a gas, producing a dense fog effect commonly used in theatrical productions and for preserving perishable goods.
2. Naphthalene (Mothballs)
Naphthalene undergoes sublimation at room temperature, releasing its distinct odor, which acts as a repellent for moths and other insects.
3. Camphor
Solid camphor sublimes at room temperature, releasing its aromatic properties and making it a popular ingredient in traditional medicines and household products.
4. Iodine
Solid iodine sublimes when heated, forming a violet-colored gas that is used in various chemical reactions and as a disinfectant.
5. Snow and Ice
Water vapor in the atmosphere can directly crystallize into ice on cold surfaces, forming snowflakes without passing through the liquid phase.
6. Frost Formation
Frost forms on surfaces when water vapor in the air directly transitions into solid ice crystals without becoming liquid first.
7. Freeze-Drying (Lyophilization)
This process involves freezing a substance and then subjecting it to reduced pressure, causing the frozen water to sublime and leaving behind a dehydrated product.
8. Air Fresheners
Many air fresheners utilize sublimation to release fragrances gradually over time, enhancing the ambiance of a space.
9. Mothproofing Agents
Sublimable chemicals like paradichlorobenzene and naphthalene are used in mothballs and mothproofing agents to protect clothing and fabrics from insect damage.
10. Printed Circuit Boards
Sublimation is used in the production of printed circuit boards to deposit metal onto non-metallic surfaces, creating conductive pathways for electronic components.
11. Sublimation Printing

This technique involves transferring dye directly onto fabrics using heat and pressure, resulting in vibrant and durable prints commonly seen in apparel and signage.
12. Phenol Sublimation
Phenol can sublime under certain conditions, making it useful in the purification of crude phenol through sublimation distillation.
13. Caffeine Purification
Sublimation is employed in the purification of caffeine, separating it from impurities and producing a highly pure form for use in beverages and pharmaceuticals.
14. Space Science
Sublimation occurs on celestial bodies like comets, where frozen gases such as water vaporize directly into space as the comet approaches the sun, creating its characteristic tail.
15. Aerosol Propellants
Some aerosol propellants, like certain fluorocarbons, undergo sublimation to propel the contents out of the canister when the valve is opened.
16. Crystal Growth
Sublimation is used in the production of semiconductor materials and optical crystals, where controlled sublimation of purified compounds results in the formation of high-quality crystals.
17. Deicing Agents
Sublimable chemicals like calcium chloride and urea are used as deicing agents to melt ice and snow on roads and walkways by absorbing heat during sublimation.
18. Perfume Testing
In perfume testing, sublimation chambers are used to release fragrances slowly, allowing testers to evaluate the scent over time and under different conditions.
19. Forensic Science
Sublimation chambers are utilized in forensic science to detect volatile compounds present in evidence, allowing for the identification of substances like drugs or explosives.
20. Food Preservation
Freeze-drying and sublimation are used in the food industry to remove moisture from foods, preserving them for longer periods while retaining flavor and nutritional value.
21. Mothproofing Paper Documents
Sublimable chemicals like paradichlorobenzene are often used in storage areas for paper documents to deter insects and protect against damage caused by pests like moths.
22. Frost-Free Refrigerators
In frost-free refrigerators, sublimation is utilized to remove frost buildup. The freezer compartment is periodically heated slightly above freezing temperature, causing accumulated frost to sublime into water vapor, which is then evaporated by a fan.
23. Asthma Inhalers

Some asthma inhalers use sublimation to deliver medication. The medication is stored as a solid within the inhaler and sublimes upon inhalation, allowing the user to inhale the medication in gas form for quick absorption into the lungs.
24. Winter Sports
In sports like snowboarding and skiing, sublimation plays a role in the formation of snow and ice on slopes. Water vapor in the air directly crystallizes into snow or ice crystals on cold surfaces, creating the ideal conditions for winter sports.
25. Sublimation in Chemistry Demonstrations
Sublimation is often showcased in chemistry demonstrations to illustrate physical changes in substances. Common examples include iodine sublimation, where solid iodine crystals transform into purple vapor upon heating.
26. Perfumed Sachets
Perfumed sachets contain aromatic substances that undergo sublimation at room temperature, releasing fragrance gradually over time. These sachets are commonly used in closets, drawers, and other confined spaces to impart a pleasant aroma.
27. Archival Conservation
In archival conservation, sublimation is employed to remove unwanted adhesives, tapes, and residues from delicate documents and artifacts. Controlled sublimation of solvents helps preserve the integrity of historical materials without causing damage.